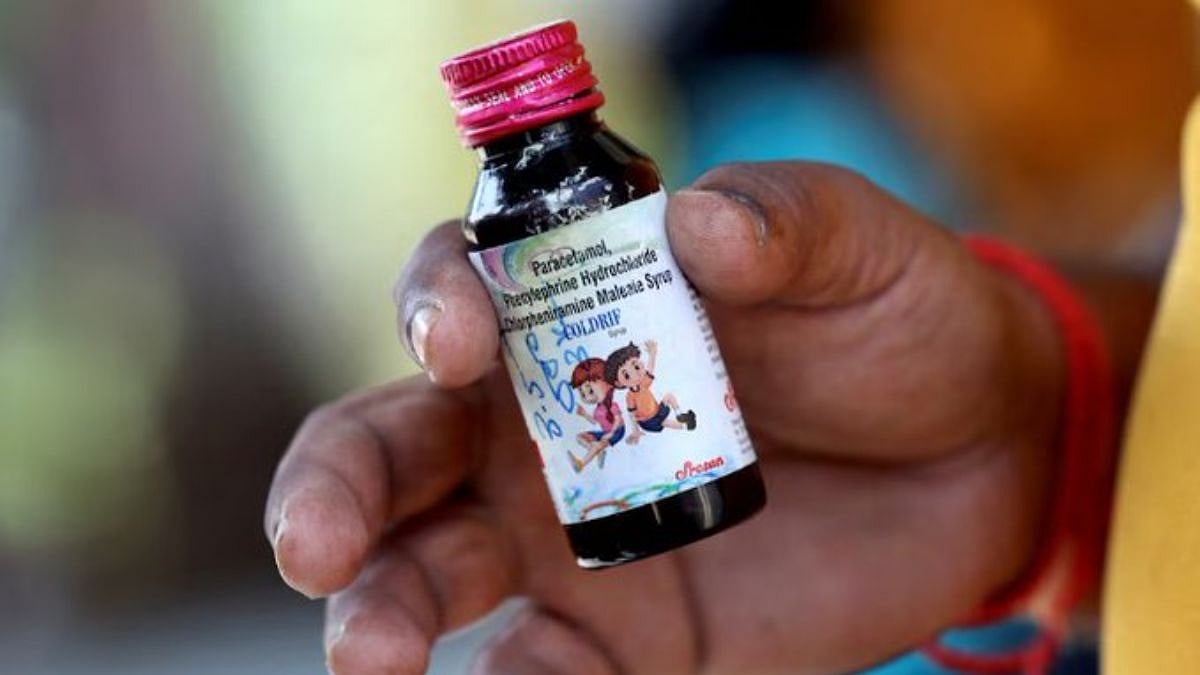
Chennai: Days after Madhya Pradesh reported child deaths linked to consumption of cough syrup manufactured by a Tamil Nadu-based pharma company, the State Drugs Control Department cancelled the manufacturing licences of Sresan Pharmaceuticals and permanently closed the factory. This followed laboratory tests, which confirmed the presence of the lethal chemical diethylene glycol in Coldrif, one of its paediatric syrups, manufactured by it.
In a related development, the Directorate of Enforcement (ED) conducted searches at the premises of the pharma company’s senior management and officials of the Tamil Nadu Drug Control Department.
The Health Department, in an official release, said Tamil Nadu had acted on an October 1 alert from the Madhya Pradesh Drugs Control Department regarding the suspected medicine, which contained paracetamol, phenylephrine hydrochloride, and chlorpheniramine maleate.
The samples of five products, including Coldrif syrup (Batch No: SR-13), were taken from the company’s Kancheepuram manufacturing plant and sent to the Government Drugs Testing Laboratory in Chennai. The analysis, completed the following day, revealed that quantities of diethylene glycol, a toxic industrial solvent, was present in the syrup.

Earlier, acting on this report, the sale of the syrup was banned in Tamil Nadu and the information was also shared with the Governments of Odisha and Puducherry, where the product had been distributed. Subsequently, a stop-production order was issued, and the company was sealed. A show-cause notice was issued to the company asking why its manufacturing licences should not be cancelled. Now the license has been cancelled.